APAC is the fast growing region globally for cell & gene therapy trials representing more than a third of all cell & gene studies globally, with China leading in the region.
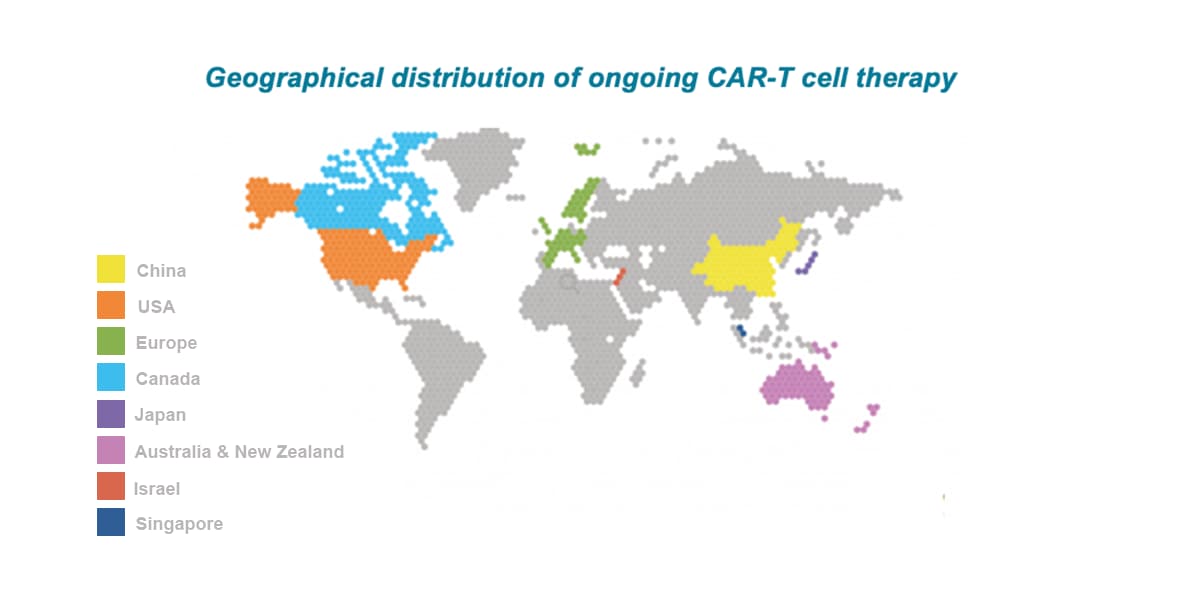
APAC is the leading location globally for CAR-T trials with China attracting ~60% of all CAR-T trials globally between 2015-2022. The number of CAR-T trials initiated by Western companies has rapidly increased in recent years (current CAGR of about 60%), with multiple targets being explored including CD19, CD20, CD22, BCMA, CD30, CD123, CD33, CD38, and CD138.
Novotech, the leading Asia Pacific biotech specialist clinical research organization with operations in APAC and the US, has extensive experience in cell & gene studies.
China has a supportive policy environment promoting a favorable cell and gene therapy ecosystem that includes:
- World-class scientific excellence
- FDA-accepted clinical trial data
- Massive patient population – which facilitates recruitment
- Government support for cell therapy trials with expedited approval processes – shortened the approval timeline to 60 working days
- Development of regional infrastructures such as cell storage and local manufacturing facilities
- Investigator-initiated cell therapy trials, which are widely conducted in China, accumulate valuable data across the different indications
- Top oncology disease treatment facilities in China deliver a practical experience in cell and gene therapy research
- While CAR-T research can be expensive, APAC costs are only about 40-50% of Western countries
- China has the world’s second-largest pharmaceutical market
- Adoption of international regulatory guidelines (ICH)
Patient Recruitment Drivers
The APAC region and specifically China show strong median patient recruitment rates and shorter enrolment periods compared to the US and Europe.
The compelling data around recruitment include:
- China shows enrollment periods 25% shorter than the US and 20% shorter than Europe.
- The APAC region shows enrollment periods almost 40% shorter than the US and 35% shorter than Europe
- China and APAC show 4 times faster median patient recruitment rates than the US and Europe.
- The APAC region shows enrollment periods almost 40% shorter than the US and 35% shorter than Europe
Oncology Cases
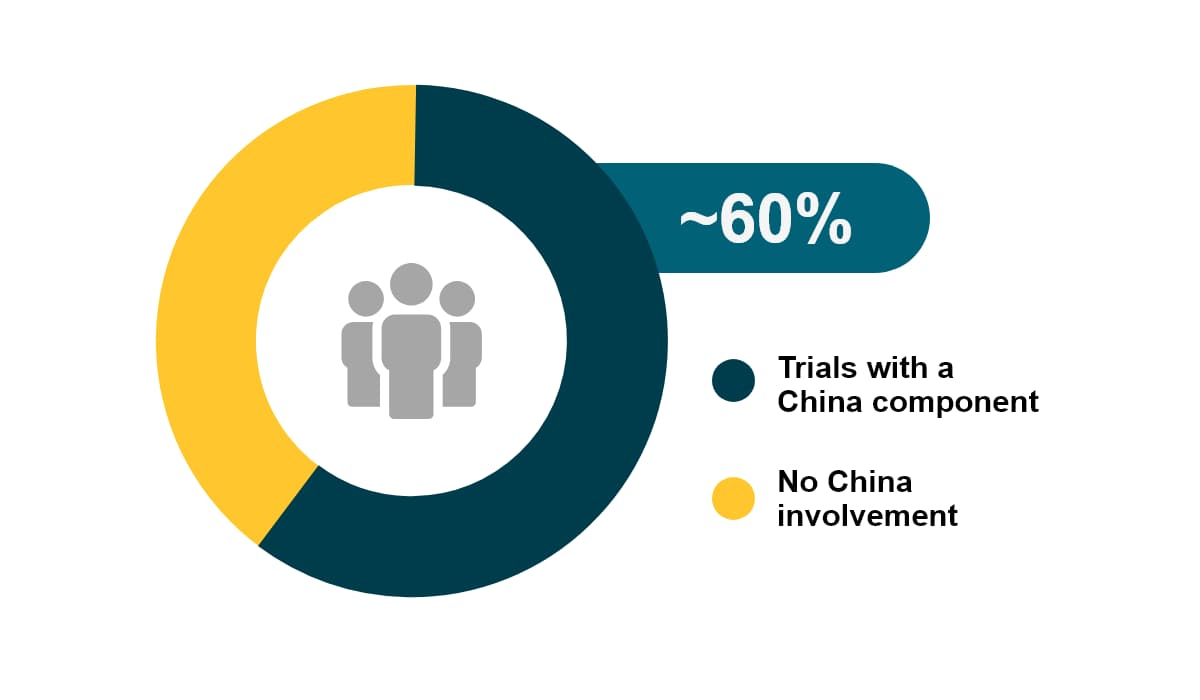
China accounts for 24% of the newly diagnosed cases globally. In 2020 China had about 4.5 million new cancer cases, as well as 30% of cancer-related deaths worldwide. Among all global CAR-T studies, about 60% have China involvement. Over the past 5 years, the growth of oncology trials (CAGR of 25%) in China outpaced all other countries.
“The number of clinical trials in China increased by 20% in 2021 and oncology represented around 40% of all clinical trials, with the majority of these being Phase 1 studies.”
Cell & Gene Therapy Landscape in APAC
Oncology trials occupy the majority of cell & gene therapy trials followed by infectious diseases, CNS, and cardiovascular diseases. Blood cancers, viral infections, and solid tumors are the major oncology indication types in cell and gene therapy trials in the Asia Pacific.
APAC shows nearly 50% faster growth rate in cell & gene therapy trials than the ROW between 2016 and 2021. China shows a 15% faster growth rate than the ROW.
Regionally, China, Australia, Japan, South Korea, and Chinese Taiwan are the leading locations participating in cell & gene therapy trials in APAC.
China Regulatory and IP Spotlight
China continues to align with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), with more than 70% of ICH guidelines now implemented. Importantly also the National Medical Products Administration (NMPA) was re-elected as a member of the ICH Management Committee in June 2021.
In addition, The Center of Drug Evaluation (CDE) guidelines on the clinical development of oncology and rare disease drugs re-emphasize patient-centric, and clinical-value-oriented development on par with ICH.
China also harmonized IP protection with global, with the new patent law effective from June 2021 research organizations.
How to include China in your clinical development strategy

Novotech has just received the AsiaPacific Cell & Gene Excellence Award 2022: Clinical Trials

媒體聯絡人
Toyna Chin
關於Novotech Novotech-CRO.com
Novotech作為一家可提供全方位服務的國際性受託研究機構 (CRO) 和科學諮詢公司,深受生物科技公司以及中小型製藥公司的信賴,引領其在各個階段的藥物開發。
公司在全球各地擁有30多個辦公據點,主要分佈在亞太地區、北美和歐洲,並與5000多個試驗機構建立了緊密的合作關係,為客戶提供豐富的臨床試驗資源網絡,幫助客戶輕鬆進軍關鍵試驗區域,廣泛接觸不同的受試者群體,推動變革性療法更快嘉惠患者。
透過以客戶為中心的服務模式,Novotech無縫整合人才、流程和技術,提供定制化解決方案,加速變革療法快速上市。透過建立緊密的合作夥伴關係,Novotech堅定不移地支援客戶實現研究目標,賦能創新,推動全球醫療健康發展。憑藉在臨床試驗執行和創新方面的卓越表現,Novotech已榮獲多項殊榮,包括連續19年蟬聯Frost & Sullivan CRO年度公司獎 (Frost & Sullivan CRO Company of the Year)。憑藉深厚的臨床領域與監管專業知識,再結合對當地市場的深刻洞察,公司致力於簡化臨床試驗流程,提升資料分析,並加快受試者招募策略的實施。
Novotech與客戶攜手並進,加速科學研發成果轉化為療法,改善全球患者結果,充分體現我們推動創新、實現卓越成果的使命。
如需瞭解更多資訊或與專家團隊成員洽談,請造訪www.Novotech-CRO.com







