APAC is the fast growing region globally for cell & gene therapy trials representing more than a third of all cell & gene studies globally, with China leading in the region.
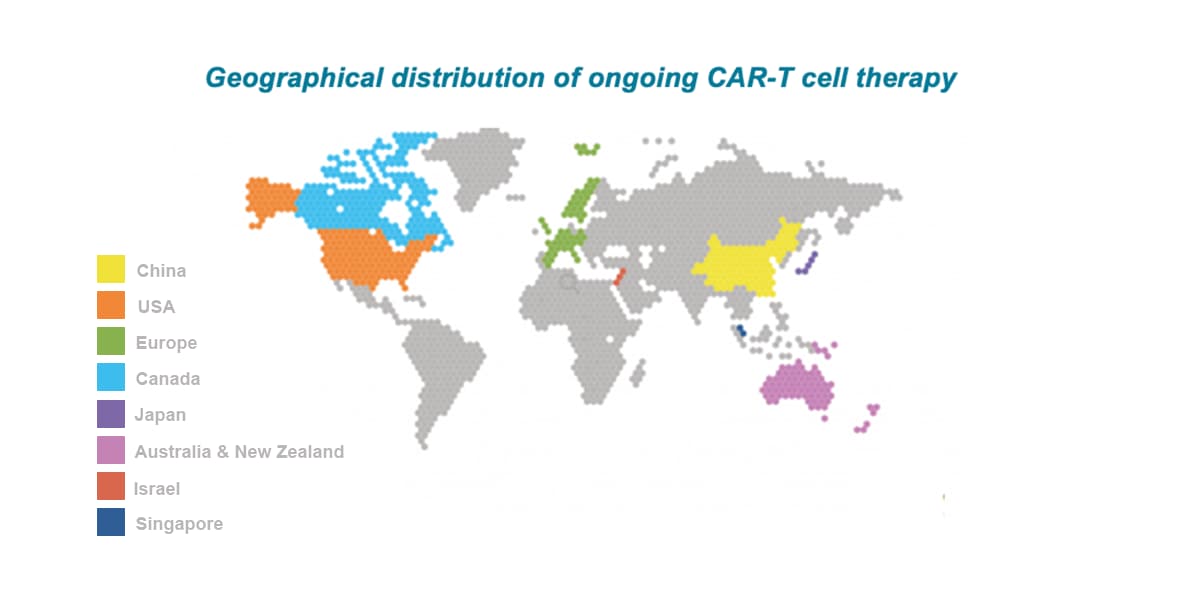
APAC is the leading location globally for CAR-T trials with China attracting ~60% of all CAR-T trials globally between 2015-2022. The number of CAR-T trials initiated by Western companies has rapidly increased in recent years (current CAGR of about 60%), with multiple targets being explored including CD19, CD20, CD22, BCMA, CD30, CD123, CD33, CD38, and CD138.
Novotech, the leading Asia Pacific biotech specialist clinical research organization with operations in APAC and the US, has extensive experience in cell & gene studies.
China has a supportive policy environment promoting a favorable cell and gene therapy ecosystem that includes:
- World-class scientific excellence
- FDA-accepted clinical trial data
- Massive patient population – which facilitates recruitment
- Government support for cell therapy trials with expedited approval processes – shortened the approval timeline to 60 working days
- Development of regional infrastructures such as cell storage and local manufacturing facilities
- Investigator-initiated cell therapy trials, which are widely conducted in China, accumulate valuable data across the different indications
- Top oncology disease treatment facilities in China deliver a practical experience in cell and gene therapy research
- While CAR-T research can be expensive, APAC costs are only about 40-50% of Western countries
- China has the world’s second-largest pharmaceutical market
- Adoption of international regulatory guidelines (ICH)
Patient Recruitment Drivers
The APAC region and specifically China show strong median patient recruitment rates and shorter enrolment periods compared to the US and Europe.
The compelling data around recruitment include:
- China shows enrollment periods 25% shorter than the US and 20% shorter than Europe.
- The APAC region shows enrollment periods almost 40% shorter than the US and 35% shorter than Europe
- China and APAC show 4 times faster median patient recruitment rates than the US and Europe.
- The APAC region shows enrollment periods almost 40% shorter than the US and 35% shorter than Europe
Oncology Cases
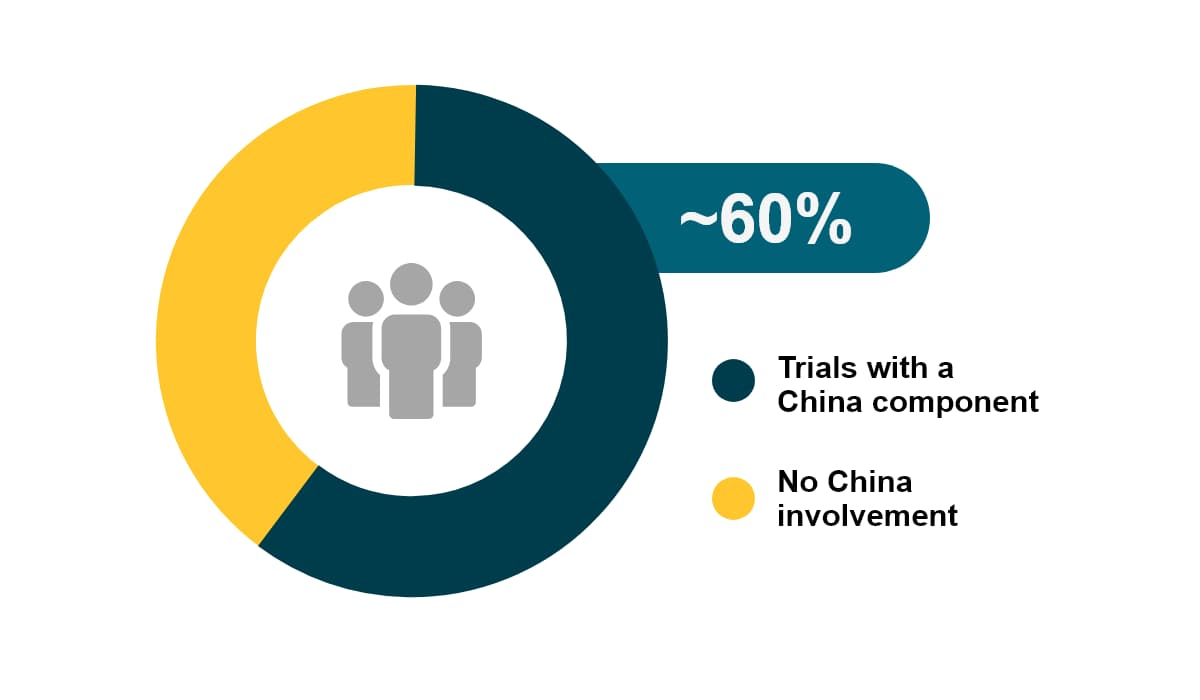
China accounts for 24% of the newly diagnosed cases globally. In 2020 China had about 4.5 million new cancer cases, as well as 30% of cancer-related deaths worldwide. Among all global CAR-T studies, about 60% have China involvement. Over the past 5 years, the growth of oncology trials (CAGR of 25%) in China outpaced all other countries.
“The number of clinical trials in China increased by 20% in 2021 and oncology represented around 40% of all clinical trials, with the majority of these being Phase 1 studies.”
Cell & Gene Therapy Landscape in APAC
Oncology trials occupy the majority of cell & gene therapy trials followed by infectious diseases, CNS, and cardiovascular diseases. Blood cancers, viral infections, and solid tumors are the major oncology indication types in cell and gene therapy trials in the Asia Pacific.
APAC shows nearly 50% faster growth rate in cell & gene therapy trials than the ROW between 2016 and 2021. China shows a 15% faster growth rate than the ROW.
Regionally, China, Australia, Japan, South Korea, and Chinese Taiwan are the leading locations participating in cell & gene therapy trials in APAC.
China Regulatory and IP Spotlight
China continues to align with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), with more than 70% of ICH guidelines now implemented. Importantly also the National Medical Products Administration (NMPA) was re-elected as a member of the ICH Management Committee in June 2021.
In addition, The Center of Drug Evaluation (CDE) guidelines on the clinical development of oncology and rare disease drugs re-emphasize patient-centric, and clinical-value-oriented development on par with ICH.
China also harmonized IP protection with global, with the new patent law effective from June 2021 research organizations.
How to include China in your clinical development strategy

Novotech has just received the AsiaPacific Cell & Gene Excellence Award 2022: Clinical Trials

Media contact
Toyna Chin
About Novotech Novotech-CRO.com
노보텍은 글로벌 full-service CRO이자 임상 과학 자문 기업으로서 모든 임상 단계에서 바이오텍 및 제약회사의 신뢰받는 파트너로 자리매김하고 있습니다.
아시아 태평양, 북미, 유럽 전역에 걸쳐 30개 이상의 지사와 5,000개 이상의 임상시험기관 파트너십을 보유하고 있으며, 다양한 환자 집단에 대한 최적의 접근성을 바탕으로 삶을 변화시키는 치료제의 성공적인 시장 진입을 지원하고 있습니다.
고객 중심 서비스 모델을 통해 노보텍은 인재, 프로세스, 기술을 유기적으로 통합하여 혁신적인 치료제를 보다 신속하게 시장에 도입하는 데 기여하고 있습니다. 또한, 진정한 파트너십 접근 방식을 바탕으로 고객의 성공, 혁신 역량 강화 그리고 전 세계 의료 발전을 향한 노보텍의 확고한 의지를 실천하고 있습니다.
노보텍은 19년 연속 Frost & Sullivan의 ‘Global Biotech CRO Award’를 비롯한 다수의 글로벌 어워드를 수상하며, 임상시험 운영 및 혁신 역량의 우수성을 공식적으로 인정받고 있습니다. 심층적인 치료 및 규제 전문성, 현지 시장에 대한 인사이트를 바탕으로 노보텍은 임상시험의 전반적인 효율성을 향상, 데이터 분석의 최적화 그리고 정밀한 환자 모집 전략 수립을 통해 고품질의 임상시험 운영을 실현하고 있습니다.
노보텍은 과학적 진보를 바탕으로, 글로벌 건강을 개선하는 혁신 치료제 개발을 실현하는 것을 사명으로 삼고 있으며, 앞으로도 바이오텍 및 제약회사의 성공적인 임상 개발 여정을 함께할 것입니다.
자세한 정보를 확인하거나 전문가와 상담하려면 www.Novotech-CRO.com을 방문하세요.







