Biometrics at Novotech
We have experience across a broad range of therapeutic areas and all phases of the drug and medical device development processes, and we can provide our customers electronic data capture solutions. We use our innovative data and analytics tools to assist our customers in data management and biostatistics for the data collected during their clinical trials. Our system can generate reports based on these data on demand, as required to meet both regulatory requirements and our customers’ marketing needs.
Our Biometrics team includes data management, database programming, statistical programming, biostatistics, and pharmacometrics, who support clinical trials across all phases.
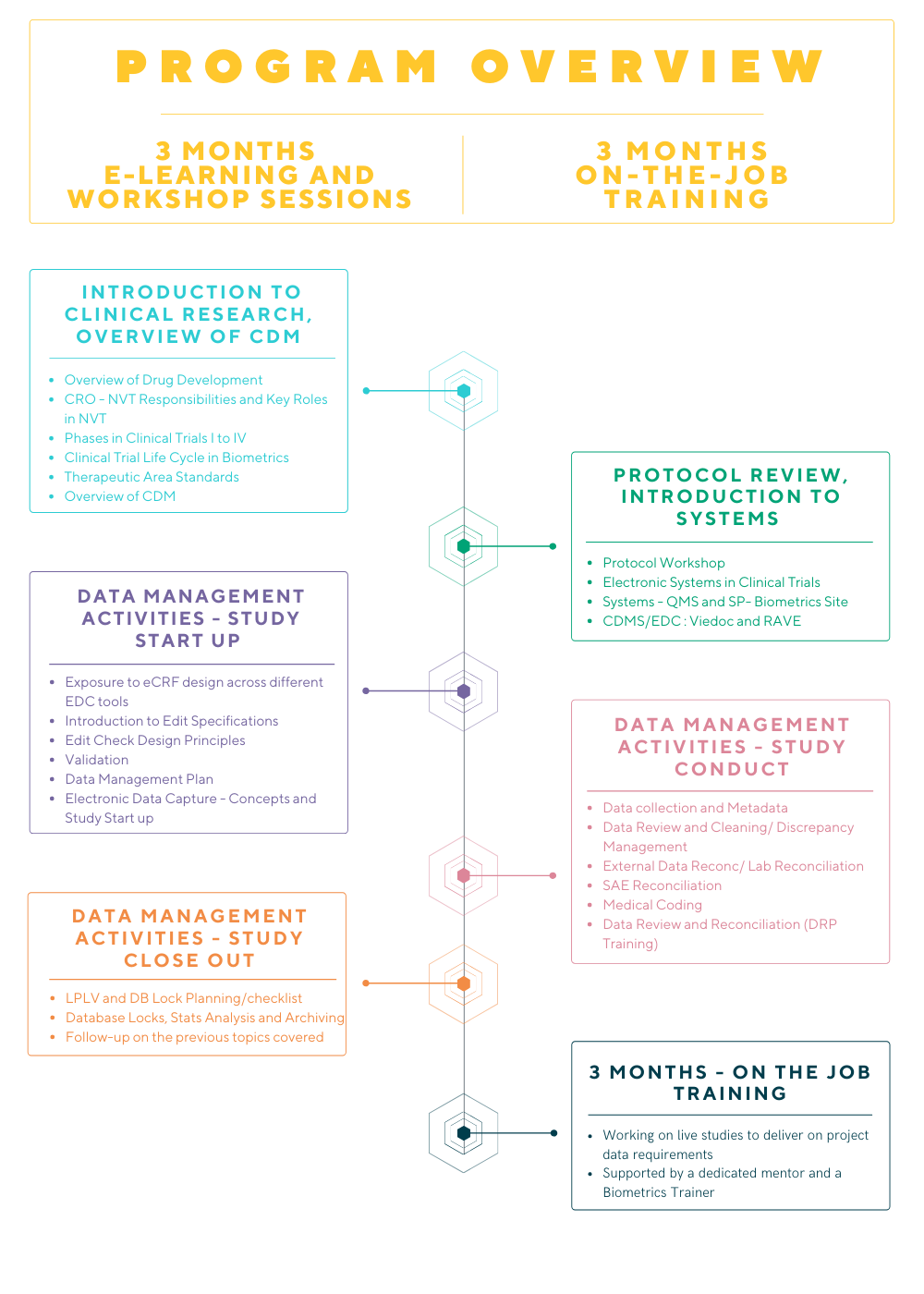
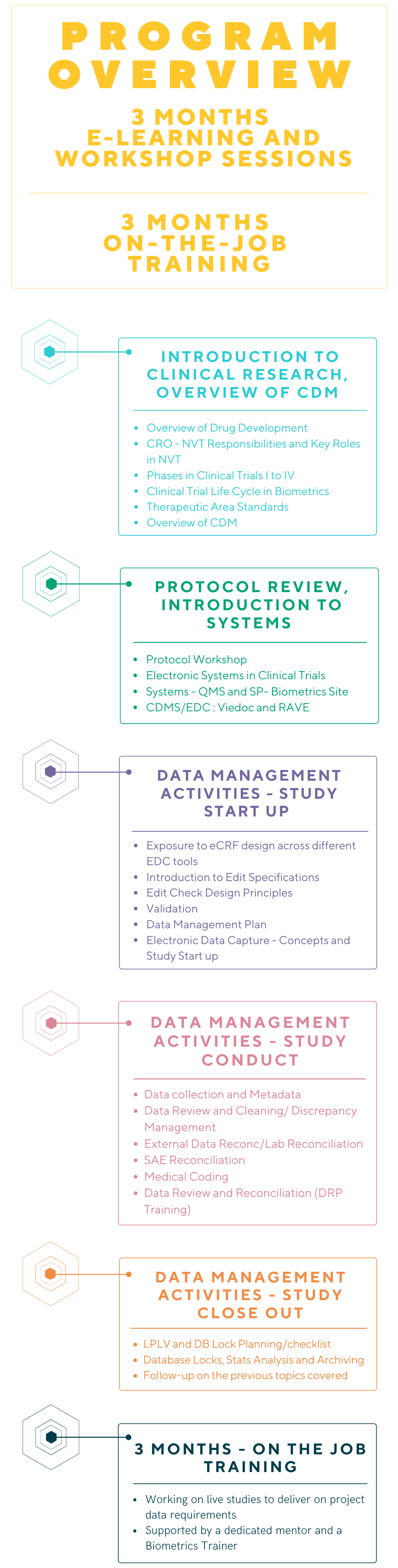
To support our continued growth and our commitment to learning & development, we have introduced the Clinical Data Programmer – SAS Trainee Program.
Clinical Data Programmer – SAS Trainee Program
As a SAS Programming Trainee, you will be a member of the Data Management Team and will perform SAS programming activities on clinical trial projects and to ensure compliance with Good Clinical Data Management Practices (GCDMP).
To be eligible for the SAS Programming Trainee Program you will need:
- Graduate in Information Technology or Pharmacy or Clinical Research or life science related field or similar.
- Base SAS Certified with good understanding of Clinical Research terminologies.
- Awareness about different electronic data capture tools and metadata would be an added advantage.