JANUARY 2022 INTAKE – AUSTRALIA AND NEW ZEALAND
We are internationally recognized as a leading regional full-service contract research organization (CRO) in Asia-Pacific providing clinical development services across all clinical trial phases and a broad range of therapeutic areas. We have created a strong benchmark, in the healthcare industry. We are a biotech focused CRO. with strong experience in a broad range of therapeutic areas including:
- Oncology
- Immunology
- Infectious Diseases
- Vaccines
- Orphan and Rare Diseases
- Neurology and Psychiatry
- Cardiovascular
- Metabolic
- Endocrinology
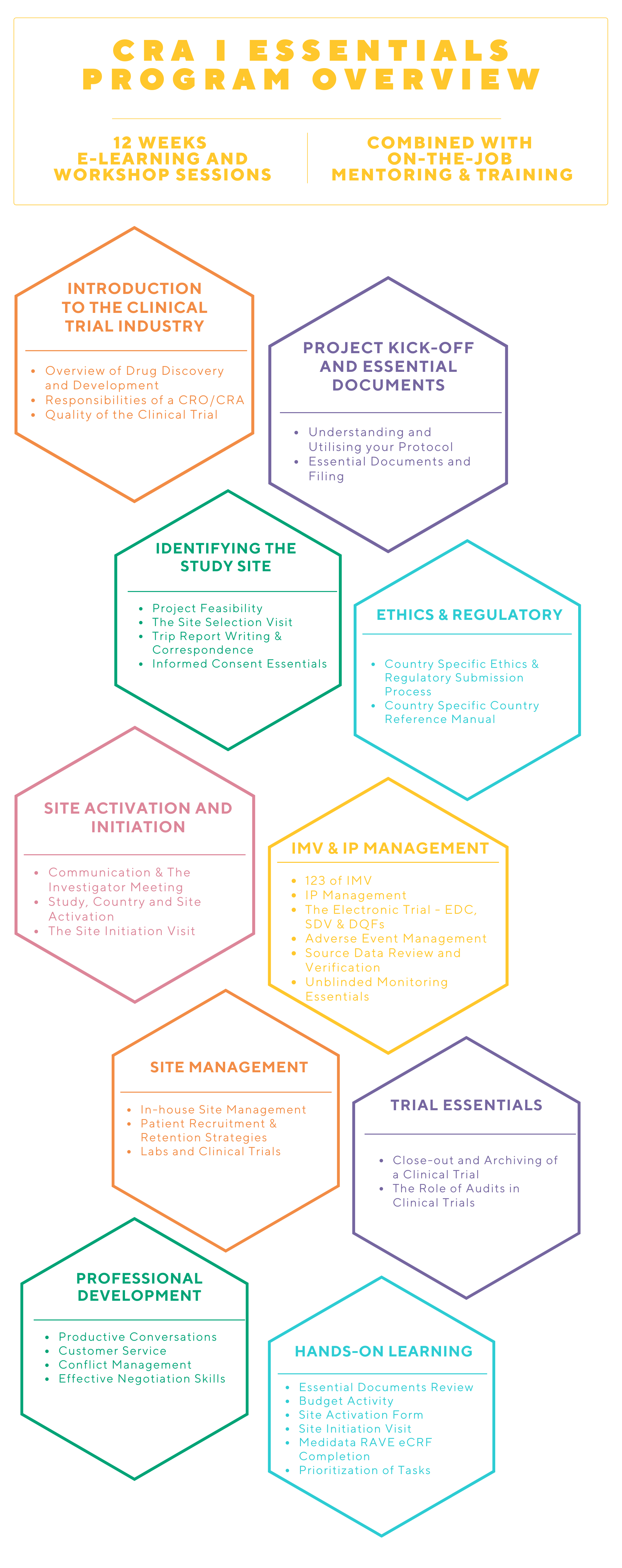
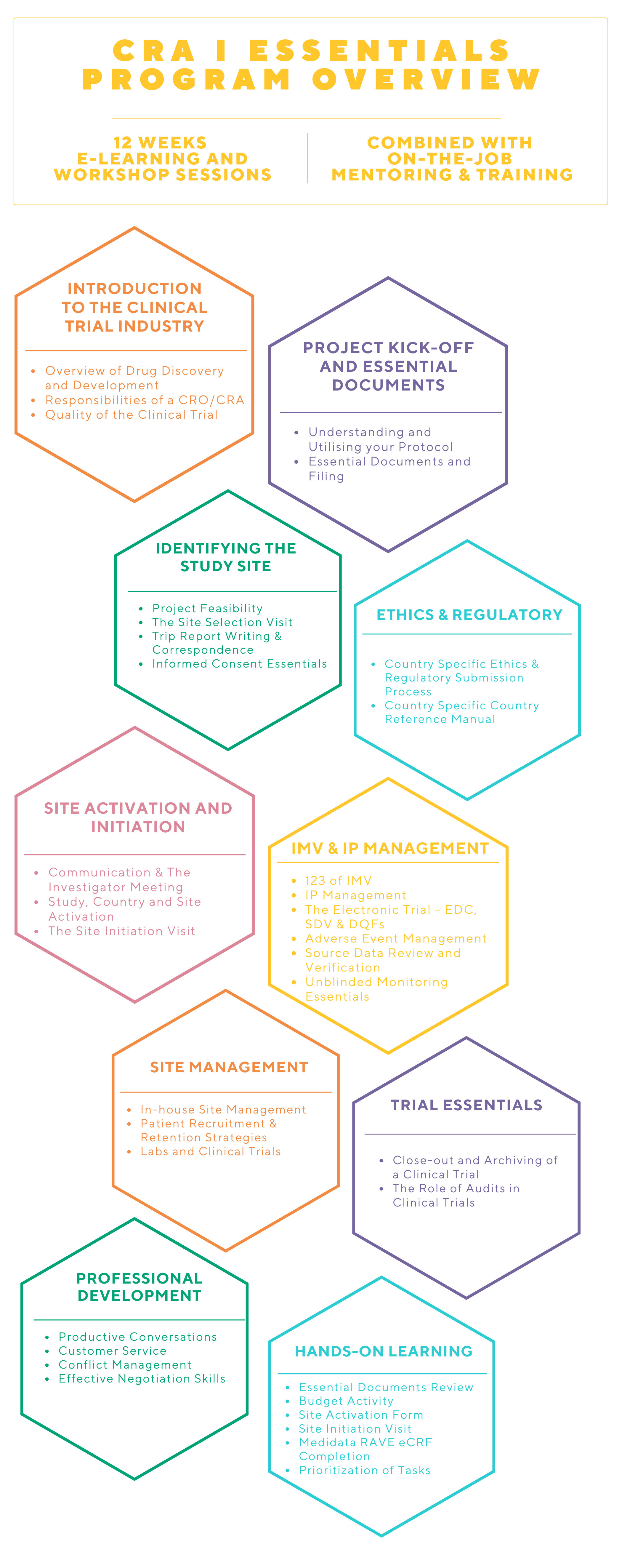
WHAT IS THE CRA I PROGRAM?
This program will provide the training and mentorship to grow your career as a Clinical Research Associate. Novotech’s CRA I program will equip you with the right site management and monitoring knowledge and skills to manage a clinical project and work alongside seasoned CRAs and Project Managers at Novotech.
This program is designed for professionals who have an education and experience in life sciences. Over 12 weeks, our CRA Essentials program is a combination of self-learning and instructor-led engagement to cover in-depth GCP and trial management learning modules with hands-on practical sessions and observation visits to build know-how and proficiency.
At the completion of the 12-week program, CRAs are assigned to projects and will continue to be mentored by our unique NovoBuddy program, Line Manager and experienced CRAs.
Apply Now
Apply Now
WHAT WILL HELP YOU SUCCEED IN THE CRA I PROGRAM?
- Your experience in the life sciences sector and knowledge of the pharmaceutical/research/ clinical trial industry will be offer advantage of familiarity with the business, research processes and interaction with customers and Investigators.
- A commitment to ongoing learning and development is essential and Novotech supports all Team Members to reach their career and personal goals.
- A passion for clinical research and improving patient outcomes.
-
HOW TO SUBMIT A GREAT APPLICATION
- Write a short cover letter
- About why you want to work in clinical trials; and
- Why you would be a great addition to our Clinical Operations Team.
- Update your CV with relevant information
- An overview of your degree and study areas; and
- A summary of your work experience including any internships related to clinical trials, academic research, pharmaceuticals or similar
- Submit your application via the Novotech Careers website here
Please note that this intake is for candidates currently located in Australia or New Zealand with full working rights. The CRAI intake in Asia will be open in early 2022.
- Write a short cover letter
OTHER OPPORTUNITIES AT NOVOTECH
Novotech also offers a variety of opportunities to join the clinical research industry, please follow us on LinkedIn for updates or check our careers site in early 2022.
- CRAI Program intake for Asia will be in March 2022, with applications open in January.
- Starting as a Clinical Trial Administrator (CTA) is a great first step into clinical research. New opportunities for CTA’s will be open in early 2022.
Apply Now