Discover the power of clinical excellence with Acrostar's SMO Division, a dedicated entity operating as part of Novotech. Since our establishment in 2013, Acrostar has emerged as a key player in the APAC clinical research realm. We've established a strong physical presence across three dynamic regions - South Korea, Taiwan, and Mainland China. With a dedicated workforce of over 300 skilled professionals, we epitomize innovation, precision, and impactful research.
Our Expertise
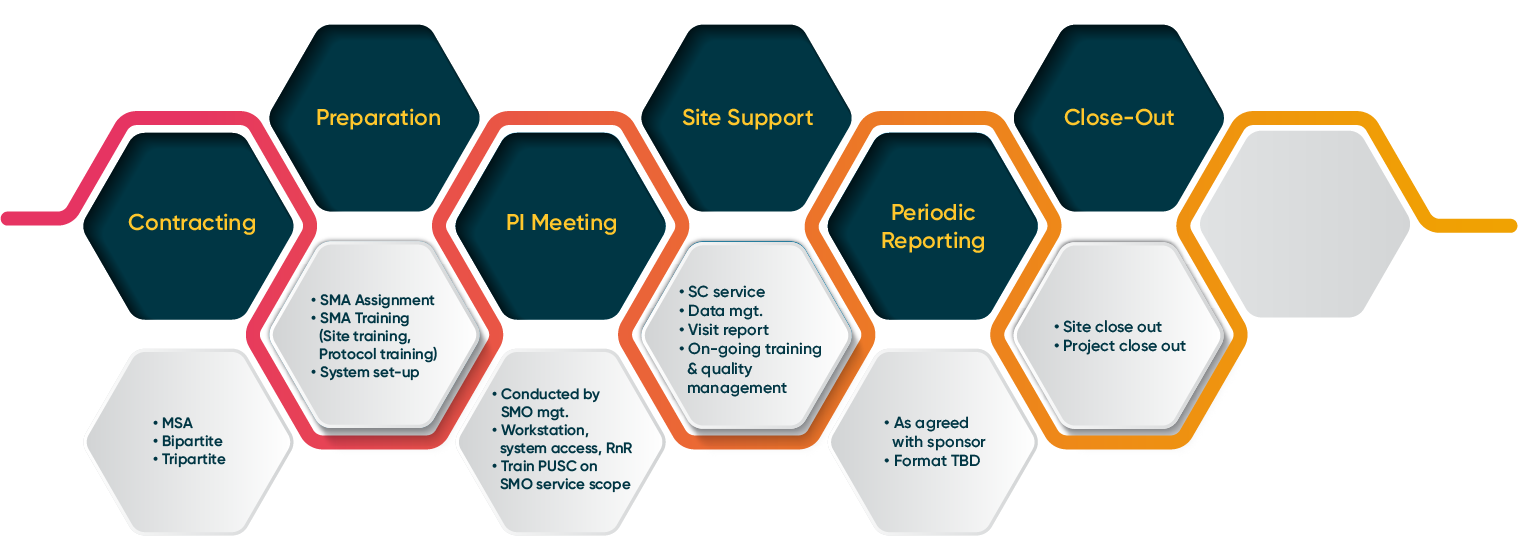
Driven by our dedication to advancing medical knowledge, SMO operates through three distinct units that collectively form the pillars of our success:
- Site Management Unit (SMU): Our proficient SMU team ensures seamless site operations, optimizing every aspect of clinical trials. From subject enrollment support and full-scope Clinical Research Coordinator service to meticulous data entry, we stand ready to streamline your study.
- Clinical Pharmacology Unit (CPU): The CPU unit, known for its agility, delivers an unmatched rapid startup process. We've perfected the art of a quick initiation, eliminating the need for hospital contracts and achieving first dosing milestones within an impressive five weeks.
- SMO Operations: With a pulse on the evolving clinical research landscape, our SMO Operations unit oversees a wide spectrum of services - from quality management, IRB assistance to AE/SAE reporting, ensuring your study's compliance and success.
Our Impact
Acrostar's achievements are quantifiable and substantial, encapsulated by:
- Studies and sites: A testament to our commitment, we've engaged in a diverse array of studies across therapeutic areas.
- Global and Local Reach: We serve both global and local clients, leveraging our expertise to propel studies on a worldwide scale.
- Phase Distribution: Our involvement spans all phases of clinical research, with an emphasis on Phase III trials (73%).
- Therapeutic Expertise: Our prowess encompasses a broad range of therapeutic areas, including oncology, pulmonology, infectious diseases, cardiology, neurology, and more.
Our Services
To optimize your clinical research journey, our SMO Division offers an extensive service portfolio:
- Scope of SC Service: Our services include meticulous schedule management, subject recruitment, AE/SAE reporting, and more, ensuring every aspect of your study aligns seamlessly.
- Scope of Site Management: From data entry to equipment management, our Site Management scope ensures the operational efficiency of your trials.
- Scope of Reporting: We assist in every aspect of reporting, from IRB initial/amendment submissions to protocol deviation reporting, guaranteeing your study's compliance.
- Contact Center Scope: With a 24-hour customer call center in the works, we offer real-time response, privacy consultation, and a comprehensive suite of subject query handling services.
Fuelling Excellence Through Partnerships
Our commitment to quality is enhanced through our robust network of site partnerships. Our collaborations drive impactful research, enabling us to deliver exceptional results and set new standards in clinical research.
For more information regarding our SMO services, please visit our website- https://acrostarsmo.com/








